BiTE®
IMMUNO-ONCOLOGY PLATFORM
THE BiTE® UNIVERSE IS EXPANDING WITH THE GOAL OF TARGETING MULTIPLE TUMOR TYPES
The BiTE® immuno-oncology platform offers versatility to potentially target any tumor-associated antigen
The BiTE® platform has the potential for off-the-shelf therapies. It is being studied across a wide range of settings, including in patients with high and low tumor burden, rapidly progressing disease, or across different treatment lines.1-3
BiTE® molecules under clinical investigation include the following targets1,4:
*Half-life extended (HLE) BiTE® platform.4
†Both canonical and HLE BiTE® platform.4
BiTE® molecules are designed to bring T-cell innovation to more patients
- Designed to target tumor-associated antigens1
- Designed to lead to off-the-shelf therapies without the need for ex vivo manipulation of patients' cells1,2
- Investigated for use as monotherapies and in combination with other treatments3,5,6
The BiTE® platform is being investigated across a broad set of cancers4
The BiTE® immuno-oncology platform has been studied in thousands of patients, many of whom have been followed for up to 5 years.7,8
With the BiTE® immuno-oncology platform, Amgen is driven to push the boundaries of science for patients with cancer by:
- Leveraging innovative trial designs9
- Investigating clinically relevant endpoints and outcomes10-12
Amgen is pioneering BiTE® technology to advance the immuno-oncology field and bring new therapeutic approaches to patients
Features of the BiTE® platform
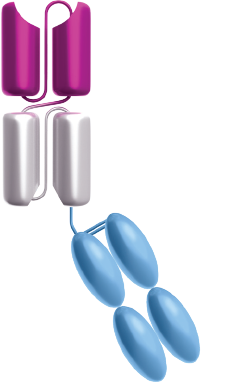
Canonical BiTE® molecules are designed to be relatively small recombinant proteins that could be cleared through the kidney, with a typical serum half-life of a few hours.3,13
Currently, Amgen is designing BiTE® molecules with additional features, including a half-life extended (HLE) BiTE® molecule containing a fragment-crystallizable (Fc) domain.14 Adding an Fc portion to the BiTE® molecule is designed to extend the amount of time before it is eliminated from the body.13
AML: acute myeloid leukemia; BiTE: Bispecific T-cell Engager; CD: cluster of differentiation; DLL3: delta-like protein 3; FLT3: FMS-like tyrosine kinase 3; GEJ: gastroesophageal junction; MRD: minimal residual disease; MUC17: mucin 17; NHL: non-Hodgkin's lymphoma; PSMA: prostate-specific membrane antigen; SCLC: small cell lung cancer.
1. Baeuerle PA, Kufer P, Bargou R. Curr Opin Mol Ther. 2009;11(1):22-30. 2. Frankel SR, Baeuerle PA. Curr Opin Chem Biol. 2013;17(3):385-392. 3. Yuraszeck T, Kasichayanula S, Benjamin JE. Clin Pharmacol Ther. 2017;101(5):634-645. 4. Amgen Pipeline. https://www.amgenpipeline.com. Accessed 4/13/2022. 5. Baeuerle PA, Reinhardt C. Cancer Res. 2009;69(12):4941-4944. 6. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02879695. Accessed 4/13/2022. 7. Data on file, Amgen; 2019. 8. Gökbuget N, Dombret H, Zugmaier G, et al. Oral presentation at: European Hematology Association Congress; June, 2019; Amsterdam, Netherlands. 9. Amgen Science. https://www.amgenscience.com/features/a-strategy-for-making-clinical-trials-more-successful. Accessed 4/13/2022. 10. Gökbuget N, Dombret H, Bonifacio M, et al. Blood. 2018;131(14):1522-1531. 11. Hoelzer D. Haematologica. 2015;100(7):855-858. 12. Harousseau JL, Avet-Loiseau H. J Clin Oncol. 2017;35(25):2863-2865. 13. Weidle UH, Tiefenthaler G, Weiss EH, et al. Cancer Genomics Proteomics. 2013;10(1):1-18. 14. Raum T, Munz M, Brozy J, inventors. US Patent 2017/0218077 A1. 8/3/2017.