HLE BiTE® technology: enhancing features of the BiTE® platform
Engineering BiTE® molecules to have a longer half-life
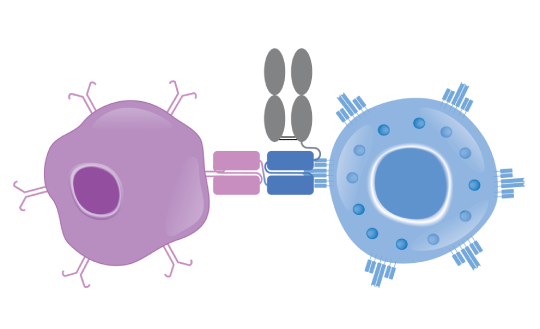
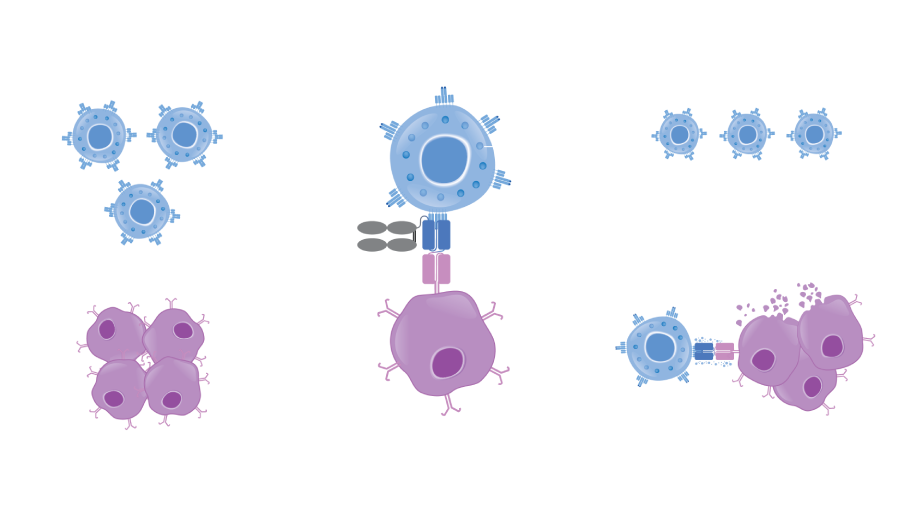
Bispecific T-cell Engager (BiTE®) technology is designed to overcome cancer cells’ evasion of the immune system by engaging patients’ own T cells to directly target cancer cells.1,2 BiTE® molecules are engineered to bind a selected tumor-associated antigen and CD3 found on T cells.3,4 Canonical BiTE® molecules are relatively small recombinant proteins that can be eliminated by the kidneys and have a typical serum half-life of a few hours.3,5
Currently, Amgen is designing half-life extended (HLE) BiTE® molecules containing a fragment crystallizable (Fc) domain.6 Adding an Fc domain to a BiTE® molecule is designed to increase the amount of time before it is eliminated from the body.7,8

Search our clinical trials.
Visit our resources section for further information on modalities currently under investigation.
Click here for more information on the BiTE® immuno-oncology platform
Extending the benefits of BiTE® technology
Canonical BiTE® molecules are relatively small recombinant proteins that can be eliminated by the kidneys and have a serum half-life of a few hours. Currently, the protein engineers at Amgen are designing BiTE® molecules with enhanced features, including a half-life extended (HLE) BiTE® molecule containing an Fc domain. Adding an Fc domain to the BiTE® molecule is designed to increase the amount of time before it is eliminated from the body.1-3,5-7
Clinical trials are underway in several cancers, including:
BiTE: Bispecific T-cell Engager; DLL3: delta-like protein 3; FLT3: FMS-like tyrosine kinase 3; GEJ: gastroesophageal junction; HLE: half-life extended; MUC17: mucin17; PSMA: prostate-specific membrane antigen.
1. Baeuerle PA, Kufer P, Bargou R. Curr Opin Mol Ther. 2009;11(1):22-30. 2. Frankel SR, Baeuerle PA. Curr Opin Chem Biol. 2013;17(3):385-392. 3. Yuraszeck T, Kasichayanula S, Benjamin JE. Clin Pharmacol Ther. 2017;101(5):634-645. 4. Nagorsen D, Baeuerle PA. Exp Cell Res. 2011;317(9):1255-1260. 5. Thakur A, Huang M, Lum LG. Blood Rev. 2018;32(4):339-347. 6. Raum T, Münz M, Brozy J, inventors. US Patent 2017/0218077A1. 8/3/2017. 7. Weidle UH, Tiefenthaler G, Weiss EH, et al. Cancer Genomics Proteomics. 2013;10(1):1-18. 8. Arvedson TL, Balazs M, Bogner P, et al. Cancer Res. 2017;77(suppl 13):Abstract 55.